Fluorine Has a Higher Ionization Energy Than Oxygen Because
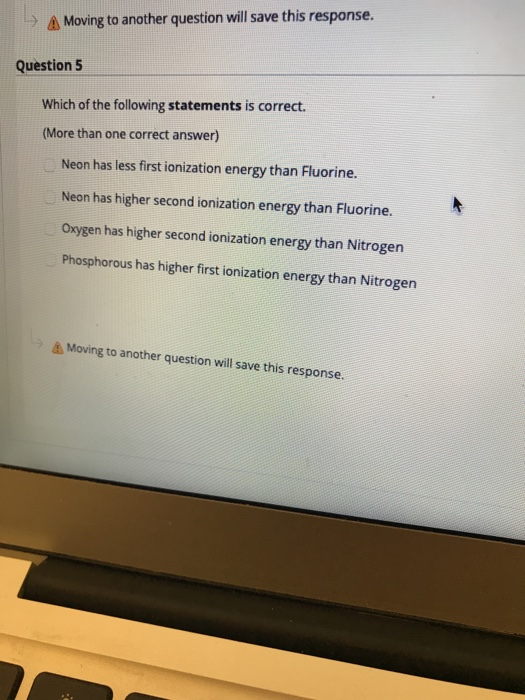
Solved A Moving To Another Question Will Save This Chegg Com

Solved Which Of The Following Statements Is Correct More Chegg Com

Although Fluorine Is More Electronegative Than Oxygen But The Ability Of Oxygen To Stabilise Higher Youtube
Why Doesnt Fluorine Have Lower Ionization Energy Than Oxygen Quora
Does Sulfur Or Oxygen Have A Higher Ionization Energy Quora

Why Is The Ionisation Enthalpy Of Oxygen Is Less Than Those Of Nitrogen Ad Fluorine

In Questions 2 4 You May Use Radius Comparisons As Given Information Ionization Energy Ionization Energy Refers Homeworklib

Solved Identify The Reasons Why Oxygen Has A Lower First Chegg Com

Unit 1 Atomic Theory And Structure

Give Reasons For The Following A Oxygen Has Lower Ionisation Energy Than That Of Nitrogen B Electron Gain Enthalpy Of Chlorine Is More Negative Than That Of Fluorine C Arrange O 2
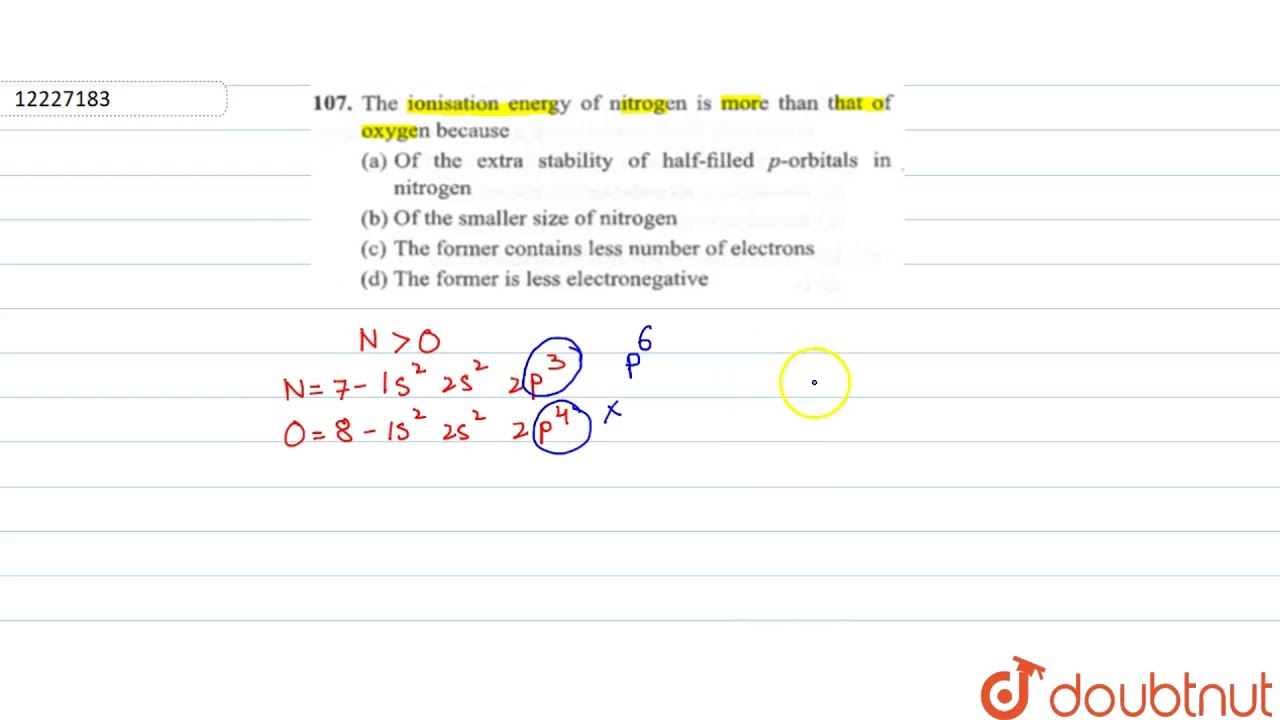
The Ionisation Energy Of Nitrogen Is More Than That Of Oxygen Because Youtube

Solved Which Of The Following Statements Is Correct More Chegg Com
Why Is The 1st Ionization Energy Of Ne Greater Than F Quora

Solved U Lonization Energy Is The Energy Needed To Remove An Chegg Com

Periodic Trends If Fluorine Has A Lower Electron Affinity Than Chlorine Why Does It Have A Higher Ionization Energy Chemistry Stack Exchange

If Oxygen Has Low Ionization Energy Because One Of The P Orbitals Is Filled With Two Electrons That Repeal Each Other Then Why Doesn T Fluorine Have Lower Ionization Energy Than Oxygen

In Questions 2 4 You May Use Radius Comparisons As Gi Itprospt

Science Chemistry Periodic Table Showme

Solved Periodicity True False Activity Group Members Your Chegg Com

Fluorine Has Lower Electron Affinity Than Chlorine Because Of Youtube
Comments
Post a Comment